The Ever Widening Scandal: The Pfizer Papers. The Company Exploited and Misapplied a Controversial Clinical Trial Method
By Dr T.J. Coles: TOTT NEWS.
Documents reveal sleight-of-hand tactics were used on clinical trial protocols to ensure the Pfizer-BioNTech vaccine was granted a license.
In April last year, the US Food and Drug Administration (FDA) published a confidential Biologics License Application guide for clinical study data reviewers.
The guide was for professionals analysing the results of large-scale tests of the Pfizer-BioNTech vaccine candidates, and the revelations are pretty shocking.
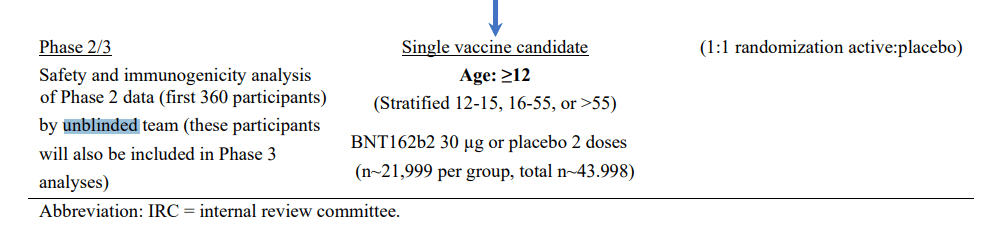
Perhaps the most blatant breach of basic clinical trial protocol was the unblinding of the safety and analysis team for Phases II and III: phases which, as we shall see, were themselves tampered with.
The document says that the first 360 participants in Phases II/III were analysed “by unblinded team” (sic).
Pre-COVID papers note, by contrast: “The gold standard clinical trial design is the double-blind, randomized, controlled trial. No standard practice exists for the ‘unblinding’ of trial participants.”
Welcome to the world of Pfizer. The unblinding has been reported elsewhere.
This article looks at the lesser-known, but equally important, revelations.
THE GUIDE LIED
— By April 2021, the Guide had been amended 12 times. Some of the amendments were added after trials had already begun, meaning that some clinical methods were altered mid-trial.
— Doses of between 20-μg and 50-μg were administered, but it is unclear whether the data were extrapolated, meaning that perhaps dose levels were mixed up, and thus efficacy hard to determine.
— Ordinarily, vaccine trials are conducted in Phases (I, II, III, VI). But the FDA originally called the Pfizer trial Stages before renaming them Phases in line with common practice.
This would suggest that the FDA trial designers knew that their methods did not constitute Phases in the traditional sense and renamed them, as not to draw attention to the flawed methods being used.
— The BNT162b2 vaccine candidate was given to participants “who originally received placebo,” meaning that it is not clear if their samples were added to the data, and if so whether BNT162b2 or their natural immunity had the given effect. A second bullet point says that some were given the BNT162b2 after the surveillance period, implying that those mentioned in the first bullet point were not.
— Appalling, in July 2020 the chronology was altered: Stages/Phases were “Renamed Stage 1 to Phase 1, removed Stage 2, and renamed Stage 3 to Phase 2/3.”
— Also in that month, patients with prior COVID-19 diagnoses were later excluded from the trials, meaning that potentially, initial natural immunity got mixed into the “vaccine” data.
— Time periods were also a hodgepodge. First, the design of Phase II/III (which according to the above actually skipped Phase II, which wasn’t even a Phase but a Stage) did not include analysis of COVID cases 14 days after the second dose. This criterion, which one would think rather crucial, was added as late October 2020. Second, in addition to the 14 days, a one-week period was later added.
Ergo, we do not know for absolutely certain who contracted what or when or whether the BNT162b2 or natural immunity played a role.
— The definition of symptoms was altered so that by October 2020 (five months into the trials), people who had two symptoms over a 4-day period were reported as having a single illness, meaning that the data could be rigged to imply that the “vaccine” reduced symptoms.
— Clinical trials typically design follow-up periods. If patients are not survielled post-vaccine, analysts cannot tell if the vaccine worked. But intensive nasal swabbing (post-vaccine) was not originally included in the assessments.
— Patients with higher risks of contracting SARS-CoV-2 (e.g., immunocompromised people, “criterion 4”) were initially excluded from surveillance “Because of a formatting error.”
SLEIGHT-OF-HAND
Even if we ignore all of the above brazen violations of vaccine trial protocol, one of the major revelations of the document is confirmation that the vaccine candidate-to-placebo ratio was inverted, compared to other trials.
It is typical to give one drug/vaccine candidate at a ratio of one placebo. This way, it is easy to tell who has been helped by the given product and who has not.
Depending on what the trial aims to determine, if symptoms are reduced and/or illness prevented more than statistical coincide in the candidate group, but not in the placebo group, the candidate can be said to be potentially effective and it will move into the next trial Phase.
It is normal to give one placebo for every product candidate.
If there are 100 participants, 50 will get the placebo. This is called a 1:1 ratio. Less common is the 4:1 ratio, which has been critiqued by clinical practitioners and analysts.
Where the 4:1 ratio is used however, it is typically used in trials with higher numbers of participants.
The FDA recommended the opposite: that the 4:1 ratio be used in the initial “Phase I” trial with fewer participants and the normal 1:1 ratio used in “Phase II/III”: the major study of over 40k participants.
In May 2020, Pfizer publicly reported: “The dose level escalation portion (Stage 1) of the Phase 1/2 trial in the U.S. will enroll up to 360 healthy subjects into two age cohorts (18-55 and 65-85 years of age).”
But the confidential FDA documents revealed that the 4:1 ratio was used.
THE OLD NORMAL
A 2011 paper reviewed the notion that trials will be “more attractive” to participants and clinicians if they include outcome-adaptive methods.
This means that if the given product does well in initial trials, more participants can be given the drug and fewer given the placebo. This is not science, but marketing.
As the authors note:“With no differential patient accrual rates because of the trial design, we find no benefits to outcome-adaptive randomization over 1:1 randomization, and we recommend the latter.”
If clinicians feel compelled to include outcome-adaptive methods, the authors recommend being limited to 2:1.
A 2014 anti-epileptic drug study (also of a Pfizer product) featured 161 participants with a 4:1 randomisation ratio. The study itself acknowledges: “unequal (4:1) randomization resulted in a small 150 mg/d [i.e., placebo] group, and results should be interpreted with caution.”
But no such cautious interpretation was afforded the Stage I, err… I mean “Phase I,” Pfizer/BioNTech trial.
A 2015 study notes: “But many RCTs [randomly-controlled trials] use unequal allocation schemes (e.g., 2:1 or 3:1), which assign more patients to the experimental intervention.”
Notice that this one does not even include 4:1, because it is so comparatively rare. Recall that this study was pre-COVID, even here the authors cautioned that outcome-adaptive methods result in “frequent discordance between effect sizes in phase 3 studies and those in phase 2.”
Even worse, in the Pfizer study, “Phase” II was lumped with III. Amazingly, the authors note that licensing success for outcome-adaptive trials is even less than the typical 50 percent that make it to Phase III.
Citing some hypothetical trial, the authors note that outcome-adaptive trials can create a sleight-of-hand that artificially boosts the efficacy percentage.
Because of statistical reduction, “their probability of being allocated to the arm that is believed to be superior is 80% rather than 50%.”
Does this sound familiar — the initial 95 percent efficacy claim made by Pfizer?
CONCLUSION
It is very, very unlikely that were it not for the pandemic, the Pfizer-BioNTech product would have been granted a license.
The dedicated work of rights advocates has given us an unparalleled opportunity to explore the Pfizer papers and unearth exactly how they, in collusion with the FDA, pulled off one of the biggest crimes of the century.
MORE TO COME
In this series, TOTT News will bring detailed analyses of the documents. We are grateful to the PHMPT for their work in bringing these pages to public light and for publishing them free on their website.
RELATED STORIES
https://asenseofplacemagazine.com/the-pfizer-papers-documents-confirm-the-vaccine-was-supposed-to-prevent-covid-not-just-reduce-symptoms/
https://asenseofplacemagazine.com/scott-morrison-endorses-handing-over-pandemic-powers-to-the-world-health-organisation/