CONFLICTS OF INTEREST in Australian Vaccination Policies
From the Informed Medical Options Party
ADVISORY COMMITTEE ON THE SAFETY OF VACCINES (ACSOV)
ROLE: "The ACSOV is established .... to advise and make recommendations to the Minister for Health (TGA) on the safety, risk assessment and risk management of vaccines."
The TGA's ACSVO has been replaced by ACV [65].
ADVISORY COMMITTEE ON VACCINES (ACV)
ROLE: "The ACV provides independent medical and scientific advice to the Minister for Health and the Therapeutic Goods Administration (TGA) on issues relating to the safety, quality and efficacy of vaccines supplied in Australia."
Chair of TGA's ACV is Allen Cheng and members include Kristine Macartney [66]. Refer to their section for COI.
Prof Cheng is also Co-chair of ATAGI [1]. Refer to ATAGI section below for COI. Director of Alfred health, which has received payments from Merck, GSK, Gilead, Biocryst and George Clinical [2].
AUSTRALIAN INFLUENZA VACCINE COMMITTEE (AIVC)
ROLE: "The AIVC provides advice to the Therapeutic Goods Administration (TGA) on the composition of the seasonal influenza vaccine to be supplied each year in Australia."
Voting Members of ATAGI's AIVC include Robert Booy and Helen Marshall [67], both of which were associated with industry-sponsored vaccine research [2], [55].
AUSTRALIAN MEDICAL ASSOCIATION (AMA)
ROLE: "Working with governments, the AMA is strategically positioned to challenge governments on policy"
Is affiliated with the Immunisation Coalition [68], which is funded by vaccine manufacturers Pfizer, CSL/Seqirus, GlaxoSmithKline, Sanofi and Merck, and Google [73].
Current President is Dr Omar Khorsahid [159].
Former President Tony Bartone [64], is a member of the Immunisation Coalition [70].
AUSTRALIAN PARTNERSHIP FOR PREPAREDNESS RESEARCH ON INFECTIOUS DISEASE EMERGENCIES (APPRISE)
ROLE: "The APPRISE Centre of Research Excellence is developing research to inform Australia’s emergency response to infectious diseases"
Their Chief Investigators include: Allen Cheng and Jodi McVernon; and Associate Investigators include: Kristine Macartney and Stephen Lambert. Refer to their sections for COI.
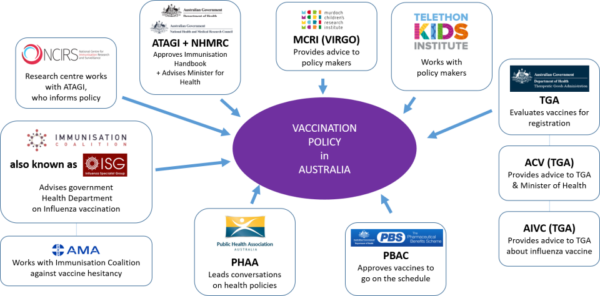
AUSTRALIAN TECHNICAL ADVISORY GROUP ON IMMUNISATION (ATAGI)
ROLE: "ATAGI advises the Minister for Health on the National Immunisation Program (NIP) and other immunisation issues."
The Australian Government National Immunisation Handbook is recommended by ATAGI [12] before being published. ATAGI advises the Minister for Health re vaccines and advises the PBAC on vaccine effectiveness.
There are currently a total of 15 members on the Advisory Group [1]. Many members of ATAGI also work on industry-funded vaccine trials. For example:
Co-chair of ATAGI is Christopher Blyth, who has received funding from Pfizer [2].
Co-chair of ATAGI is Allen Cheng, Director of Alfred health, which has received payments from Merck, GSK, Gilead, Biocryst and George Clinical [2], which are all involved in vaccine manufacturing.
Member of ATAGI is Nigel Crawford who is employed by Murdoch Children's Research Institute [1] & [2] which has received funding from Vaccination and Immunisation Research Group by vaccine manufacturers GSK, Janssen, Merck, Novavax, Sanofi and Sequiris [71].
Member of ATAGI, Michelle Giles, is an infectious disease Physician, who has received travelling, accommodation and registration to a vaccine conference from Pfizer [4]. She also received grants from Monash University [1].
Member of ATAGI, Tom Snelling is Head of Infectious Disease and Implementation Research of Telethon Kids Institute [1], which is partnered with Johnson & Johnson, Roche, Pfizer, Novartis, GSK and Sanofi [88]. He is also on the committee of the PBAC [85].
IMMUNISATION COALITION
ROLE: "We collaborate with like-minded organisations such as Primary Health Networks (PHNs), Public Health Units, Government health departments and other groups that fight vaccine hesitancy."
Receives funds from vaccine manufacturers Pfizer, CSL/Seqirus, GlaxoSmithKline, Sanofi, Merck, and Google [73].
Their special interest group, Influenza Specialist Group (72), received funds from vaccine manufacturers Abbott, GSK, Seqirus, Pfizer, Roche and Sanofi [69].
The Immunisation Coalition is also associated with the Australian Medical Association [AMA], the Royal Australian College of General Practitioners and other medical organisations [68].
Board of Directors of the Immunisation Coalition is Robert Booy. Members include Margie Danchin and former AMA President, Tony Bartone [70]. Refer to their section for COI.
Chairman, Rod Pearce [37], is also a member of the Asia-Pacific Alliance for the Control of Influenza (APACI) [32] which received funds from vaccine manufacturers IFPMA, Roche and Seqirus/CSL [34].
CEO, Mr Kim Sampson [38], is also the Executive Director of the Asia-Pacific Alliance for the Control of Influenza (APACI) [33] which received funds from vaccine manufacturers IFPMA, Roche and Seqirus/CSL [34].
INFLUENZA SPECIALIST GROUP (ISG)
ROLE: "ISG is a Special Interest Group of the Immunisation Coaltion,"
Received funds from vaccine manufacturers Abbott, GSK, Seqirus, Pfizer, Roche and Sanofi [69].
ISG is a special interest group of the Immunisation Coalition [72] which received funds from vaccine manufacturers GSK, Merck, Pfizer, Sanofi and Seqirus/CSL and Google [73].
Their annual meetings include government health officials and pharmaceutical industry representatives [74].
MURDOCH CHILDREN'S RESEARCH INSTITUTE (MCRI)
ROLE: "MCRI research contributes to changes in policy and practice in Australia and around the world."
MCRI is involved in developing new vaccines and testing [75].
MCRI is in collaboration with Vaccination and Immunisation Research Group which has received funding from vaccine manufacturers GSK, Janssen, Merck, Novavax, Sanofi and Sequiris [71].
Based on their 2019 Annual report, they have received support from Billl And Melinda Gates Foundation & Pfizer [152].
In 1984, Rupert Murdoch and family donated $5 million to The Birth Defects Research Institute at Melbourne's Royal Children's Hospital which would then be renamed Murdoch Institute for Research into Birth Defects [76].
Rupert Murdoch's mother Dame Elisabeth Murdoch then became a founding member of the MCRI [77] and in 2014, Sarah Murdoch became a member of the Board and the Ambassador for MCRI [130].
News Corp Australia is a corporate partner of the MCRI [78]. Tabloids were behind the "No Jab, No Play" media campaign [79], which was obligingly adopted as policy by politicians across the political spectrum in Australia, and enacted as the coercive "No Jab, No Pay" law under Malcolm Turnbull as Prime Minister in January 2016. And in 2016, News Corp Australia acquired Sky News channels in Australia [153].
NATIONAL CENTRE FOR IMMUNISATION RESEARCH & SURVEILLANCE (NCIRS)
ROLE: "to inform policy and planning for immunisation services in Australia".
Core funding for NCIRS is provided by Australian Government [131] and plays a major role in supporting ATAGI and its working parties [80].
Former Director of NCIRS (2005 to 2017), Peter McIntyre, was associated with industry-sponsored vaccine research [4].
Former Head of Clinical Research at NCIRS (2005 to 2019), Robert Booy [54]. Refer to his section for COI.
AusVaxSafety is Australia's active vaccine safety system, led by NCIRS [156]. NCIRS operations and research activities are overseen by an Advisory Board and a Scientific Advisory Committee.
Board Members of the Advisory Board include Jodie McVernon, Kristine Macartney, Stephen Lambert and Terry Nolan [125]. Refer to their section for COI.
Board Members of the Scientific Advisory Committee include Stephen Lambert (Chair), Helen Marshall, Peter Richmond, Tom Snelling and Margie Danchin [133]. Refer to their section for COI.
Sharing Knowledge About Immunisation (SKAI), setup by NCIRS to facilitate conversations about childhood immunisation between parents and healthcare providers [157]. It was in partnership between researchers from the University of Melbourne, Murdoch Children’s Research Institute, and Telethon Kids Institute [158]. Refer to their sections for COI. The team at SKAI included Margie Danchin, Kristine Macartney, Tom Snellingand lead by Terry Nolan. Refer to their sections for COI.
In 2016, NCIRS disclosed its clinical research group undertakes a mix of investigator driven and industry sponsored research including studies supported by vaccine manufacturers [81].
NATIONAL HEALTH & MEDICAL RESEARCH COUNCIL (NHMRC)
ROLE: "NHMRC became an independent statutory agency within the portfolio of the Australian Government Minister for Health and Ageing,"
The Australian Government National Immunisation Handbook is approved by NHMRC [12] before being published.
The Chairperson of the NHMRC is Bruce Robinson [10], who was formerly on the Advisory Board for AstraZeneca and Bayer Australia [10] & [11].
Anne Kelso is the CEO of NHMRC [6]. Refer to her section for COI.
The Medical Journal of Australia (MJA) published in 2011 “Only 15% of guidelines on the NHMRC portal from the most prolific developers have published conflict of interest statements, and fewer detail the processes used to manage conflicts” [82].
PHARMACEUTICAL BENEFITS ADVISORY COMMITTEE (PBAC)
ROLE: "The PBS Schedule lists all of the medicines available to be dispensed to patients at a Government-subsidised price".
The PBS is managed by the Department of Health [83].
The PBAC’s Cost Recovery Fees & Charges involves the Australian Government charging the non-government sector for some or all of the efficient costs of a specific government activity. That activity may include the provision of goods, services or regulation, or a combination of them. [84].
Member of PBAC, Tom Snelling [85], is also Head, Infectious Disease Implementation at Telethon Kids [86], which is partnered with Johnson & Johnson, Roche, Pfizer, Novartis, GSK and Sanofi [88].
PUBLIC HEALTH ASSOCIATION OF AUSTRALIA (PHAA)
ROLE: "We lead the conversation in public health policy across Australia".
The PHAA state that they lead the conversation in public health policy across Australia. They work collaboratively with groups to produce evidence-based policy positions to improve public health in Australia [89].
The PHAA run the National Immunisation Conference every 2 years which is sponsored by vaccine manufacturers. e.g. 2021 conference sponsored by GSK and Seqirus [90]; and in 2016 sponsored by GSK, Seqirus, Sanofi and Pfizer [91].
During their 2014 conference, the PHAA promoted a poster produced by Stop the Australian Vaccination Network, a coercive vaccination lobby group [92].
RESET AUSTRALIA
ROLE: "We work exclusively in Australia on public policy advocacy".
Reset Australia is a global initiative working to counter digital threats to democracy [143]. They claim to work exclusively in Australia on public policy advocacy, research, and civic engagement to strengthen our democracy.
The coalition, led by Reset Australia, is working against misinformation regarding COVID-19 vaccination efforts [144]. The coalition includes:
The Immunisation Coalition, which received funds from vaccine manufacturers GSK, Merck, Pfizer, Sanofi and Seqirus/CSL and Google [73];
The Immunisation Foundation of Australia.
Coronavax a project led by Chris Blyth based at the Telethon Kids Institute [145], which is partnered with Johnson & Johnson, Roche, Pfizer, Novartis, GSK and Sanofi [88];
The Doherty Institute, who are in collaboration with VIRGo [71]. Refer to their section below for COI.
TELETHON KIDS INSTITUTE
ROLE: "We bring together community, researchers, practitioners, policy makers and funders".
Partnered with Johnson & Johnson, Roche, Pfizer, Novartis, GSK and Sanofi [88].
THERAPEUTIC GOODS ADMINISTRATION (TGA)
ROLE: "The TGA is part of the Australian Government Department of Health, and is responsible for regulating therapeutic goods".
The TGA is the Australian government regulator of therapeutic goods such as medicines and vaccines, which evaluates and registers products [93].
The funding system they use is known as Cost Recovery (or User-Pay) and it means that the TGA recovers the full cost of its regulatory activities by charging the sponsors and manufacturers of the products that are regulated [94].
The pharmaceutical and manufacturing industry funds the TGA even though this government body has the dual role of approving drugs for its sponsor and monitoring the safety of these same drugs in the Australian population.
VACCINE & IMMUNISATION RESEARCH GROUP (VIRGo)
ROLE: "Our findings enable us to advise policy makers on the optimal use of vaccines in national immunisation schedules".
VIRGo is a collaboration between the Murdoch Children's Research Institute and the Peter Doherty Institute for Infection and Immunity at the University of Melbourne [71].
VIRGo has received funds from vaccine manufacturers GSK, Janssen, Merck, Novavax, Sanofi and Sequiris [71].
Head of VIRGo is Terry Nolan [57]. Refer to section Terry Nolan for COI.
Currently leads vax4COVID, which is an alliance of experienced Australian vaccine clinical trial centres formed to facilitate the conduct of Phase II trials of SARS-CoV-2 vaccine candidates [71].
INDIVIDUALS
Dr Katie ATTWELL
Member of Immunisation Coalition [70], which is funded by vaccine manufacturers Pfizer, CSL/Seqirus, GlaxoSmithKline, Sanofi and Merck and Google [73].
Part of a team conducting an evaluation of the "I Immunise" campaign, funded through an unrestricted grant from vaccine manufacturer Sanofi-Pasteur [36].
Prof Ian BARR
Deputy Directorof the WHO Collaborating Centre for Reference and Research on Influenza at the Doherty Institute, where he has had over 35 years’ experience in biological research and development in academic institutions and at CSL on various topics such as vaccine development [17].
From 2015 to 2016 and in 2007, was on the Advisory Group of Experts for WHO Consultations on the Composition of Influenza Virus Vaccines [137].
Member of Immunisation Coalition [70], which is funded by vaccine manufacturers Pfizer, CSL/Seqirus, GlaxoSmithKline, Sanofi and Merck and Google [73].
Dr Tony BARTONE
Former President of the Australian Medical Association [64] (resigned late 2020 after 2 years).
Member of Immunisation Coalition [70], which is funded by vaccine manufacturers Pfizer, CSL/Seqirus, GlaxoSmithKline, Sanofi and Merck and Google [73].
A/Prof Christopher BLYTH
Co-Chair of Australian Technical Advisory Group on Immunisation (ATAGI) [1]. Refer to ATAGI section for detailed COI.
Investigator on a pneumonia/meningitis study in Papua New Guinea. Grant received by employer (The University of Western Australia) from Pfizer [2].
Co-Chair of ATAGI's COVID-19 Working Group [148] which provides advice to the Minister for Health on the immunisation program for COVID-19 vaccines as they become available in Australia.
Member of COVID-19 Vaccine & Treatments for Australia – Scientific & Industry Technical Advisory Board [3]
Co-Director of Wesfarmers Centre for Vaccines and Infectious Diseases; Telethon Kids [147], which is partnered with Johnson & Johnson, Roche, Pfizer, Novartis, GSK and Sanofi [88].
Prof Robert BOOY
Medical Advisor of Meningococcal Australia [52], which has received funds from vaccine manufacturers GSK and Pfizer [53].
Was Head of Clinical Research at the National Centre for Immunisation Research & Surveillance 2005 to 2019 [54].
Previously Chair, currently Director and Scientific Advisory Member of the Immunisation Coalition [37], which is funded by vaccine manufacturers Pfizer, CSL/Seqirus, GlaxoSmithKline, Sanofi and Merck and Google [73].
In 2009, Robert Booy was a co-author of an "Influenza Vaccine trial" which was funded by CSL [55].
He received funding from CSL, Roche, Sanofi Pasteur, GSK and Pfizer to conduct research or attend and present at scientific meetings [55].
Member of Friends of Science in Medicine [95], which supported "No Jab, No Pay" at the Senate Hearing in Nov 2015 [16].
A/Prof Julia BROTHERTON
Co-Author of the Department of Health’s report “Planning for human papillomavirus vaccines in Australia”. This is the report of a meeting convened by the National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases on 12 December 2003, bringing together representatives from eight Australian research groups involved in HPV research, and vaccine manufacturers with HPV vaccine candidates. The meeting was supported by vaccine manufacturers CSL Pharmaceuticals and Glaxo SmithKline [35].
Prof Allen CHENG
Director of the Infection Prevention and Healthcare Epidemiology unit at Alfred Health [140], which has received payments from vaccine manufactureres Biocryst, George Clinical, Gilead, GSK and Merck [2], and has an appointment in the School of Public Health and Preventive Medicine at Monash University [140].
Co-Chair of ATAGI [1]. Refer to ATAGI section for detailed COI.
Member of the COVID-19 Vaccine & Treatments for Australia – Scientific & Industry Technical Advisory Group [3].
Co-Chair of ATAGI's COVID-19 Working Group [148] which provides advice to the Minister for Health on the immunisation program for COVID-19 vaccines as they become available in Australia.
Deputy Chief Health Officer of Victoria [126] and influential on coronavirus policy.
Therapeutic Goods Administration (TGA) Advisory Committee on infectious diseases and biostatistics [3]. Refer to TGA section for detailed COI.
Site Investigator for a number of infectious disease studies via Alfred Health, which has received payments from vaccine manufactureres Biocryst, George Clinical, Gilead, GSK and Merck [2].
Chief Investigator for influenza surveillance (FluCAN Surveillance System) [3].
In 2018 Chair of the Influenza Working Party which prepares advice for ATAGI [30].
His team has recently received $1.7M from federal governments National Health & Medical Research Council (NHMRC), Refer to NHMRC section for COI, towards research into how influenza virus infection results in severe illness and death in Australia [43].
Peter COSTELLO
Chairman of the Board of Guardians of the Future Fund [161], an Australian government investment fund, with over 2 billion dollars worth of shares in pharmaceutical and vaccine manufacturers i.e. CSL, Johnson and Johnson, Pfizer, Novartis and Merck [100]. The Future Funds investment in pharmaceutical and vaccine manufacturers has doubled from $1 billion to $2 billion during the coronavirus situation.
Non-Executive Chairman of Nine Entertainment Co. [44].
Nine Entertainment own newspapers "the Sydney Morning Herald" and "The Sun Herald" [154], who were/are instrumental in "anti-vax" labelling [155].
A/Prof Nigel CRAWFORD
Currently Director of Surveillance of Adverse Events Following Vaccination in the Community (SAEFVIC) at Murdoch Children's Research Institute [42], which has received funding from Vaccination and Immunisation Research Group from Novartis Vaccines, GSK and Sanofi [71] & [4].
In 2014 was appointed to ATAGI [42].
Member of ATAGI's COVID-19 Working Group [148] which provides advice to the Minister for Health on the immunisation program for COVID-19 vaccines as they become available in Australia.
Previously Director of The Influenza Specialist Group [2], which received funding from vaccine manufacturers Abbott, GSK, Seqirus, Pfizer, Roche and Sanofi [69].
A/Prof Margie DANCHIN
Member of Immunisation Coalition [70], which is funded by vaccine manufacturers Pfizer, CSL/Seqirus, GlaxoSmithKline, Sanofi and Merck, and Google [73].
Group Leader of Vaccine Updake at Murdoch Children's Research Institute (MCRI) [41]. Refer to MCRI section for COI.
She is also promoting COVID-19 vaccination amongst elderly and children [127].
Laureate Prof Peter DOHERTY
The Doherty Institute for Infection & Immunity is named in honour of Patron, Laureate Professor Peter Doherty, winner of the 1996 Nobel Prize [45], where Peter Doherty is still active in science and involved in large, grant-funded programs at both institutions, he commutes between St Jude Children’s Research Hospital in Memphis and the Doherty Institute where he now spends most of his time [134].
The Peter Doherty Institute is also in collaboration with Vaccine and Immunisation Research Group/Murdoch Children's Research Institute [71] which has received funding from vaccine manufacturers GSK, Janssen, Merck, Novavax, Sanofi and Sequiris [71].
The Peter Doherty Institute have released their work on COVID-19 modelling to the general public. These models have been utilised by the Commonwealth Government in the public health response to COVID-19 [138].
Peter Doherty is a member of Friends of Science in Medicine [95], which supported "No Jab, No Pay" at the Senate Hearing in Nov 2015 [16].
A/Prof Katie FLANAGAN
Is affiliated to Monash University [141].
In the last 10 years has been involved in research projects awarded in excess of AU$30 million, includes grants from NHMRC and Bill and Melinda Gates Foundation [142]. Refer to their sections below for COI.
Former member of Influenza Vaccine Advisory Board, travel paid for by vaccine manufacturers Sanofi and Seqirus [3].
Member of ATAGI's COVID-19 Working Group [148] which provides advice to the Minister for Health on the immunisation program for COVID-19 vaccines as they become available in Australia.
Presenter at a number of conferences in clinical infectious diseases sponsored by vaccine manufacturer Pfizer [3].
Former member of a number of vaccine advisory boards for the influenza vaccine [3].
Prof Ian FRAZER
Co-Inventor of Gardasil vaccine against HPV, (patent with vaccine manufacturer CSL and The University of QLD) [13] and he receives royalties from HPV vaccine sales in the developed world [18].
Member of Friends of Science in Medicine [95], which supported "No Jab, No Pay" at the Senate Hearing in Nov 2015 [16].
Jane HALTON
Adviser to Prime Minister Scott Morrison on the government’s National COVID-19 Coordination Commission [19].
She is Chair of the Coalition for Epidemic Preparedness Innovations (CEPI) [20], which has funded $388M to COVID-19 vaccine manufacturer Novavax [21], which is being trialled in Australia [22].
She is pushing for COVID "No Jab, No Play" policy to be adapted to adults [23].
She is Director of Crown Resorts [24], which is the COVID-19 quarantine facility in Victoria [25].
Her husband, Trevor Sutton, was the Deputy for Australian Statistician of the Australian Bureau of Statistics (ABS) and sits on the advisory committee of Institute for Health Metrics & Evaluation (IHME) [26] of which Jane Halton sits on the board [27]. In 2007, the IHME received funding from Bill & Melinda Gates Foundation [46].
In 2016, The ABS’s data collection and survey systems was awarded to Accenture [28], which is in direct partnership with ID2020 agenda and GAVI [29]. Refer to GAVI section for COI.
Dr David HAWKES
Is the Director - Meleculator Biology and Viochemistry of VCS Foundation (Victorian Cytology Service)[8], which promotes HPV vaccination.
The VCS research centre, known as C4, receives funds from National Health & Medical Research Council [9]. Refer to NHMRC section for COI.
Member of Friends of Science (FSM) [95] which supported "No Jab, No Pay" bill at the Senate Hearing in Nov 2015 [16].
Administrator with Stop the Australian Vaccination Network (SAVN) [92], [128], which supported "No Jab, No Pay" Senate Inquiry Hearing in 2015 [97].
Prof David Isaacs
Was a member of government's Pharmaceutical Benefits Advisory Committee [162], refer to PBAC section for COI.
During that time, co-author of journal "Introducing a new group B meningococcus vaccine" [162].
Prof Anne KELSO
Collaborates with WHO Influenza and Essential Regulatory Laboratories [5].
CEO of National Health & Medical Research Council [6]. Refer to NHMRC section for COI.
From 2007 to 2015 was on the Advisory Group of Experts for WHO Consultations on the Composition of Influenza Virus Vaccines [137].
Had an undisclosed number of shares in vaccine manufacturer CSL where she claimed she bought well before 2007 [7].
In 2009, she helped draw up Australia's response to the swine flu outbreak [7]. CSL was given a $131 million federal government contract to produce the nation's entire stockpile of swine flu vaccine in 2009 [7].
Dr Omar KHORSHID
Current President of the Australian Medical Association [159].
The AMA is affiliated with the Immunisation Coalition [68], which is funded by vaccine manufacturers Pfizer, CSL/Seqirus, GlaxoSmithKline, Sanofi and Merck and Google [73].
Omar has stated "vaccinating the whole of the population is the only way “to survive” coronavirus coming to our shores". {160].
Dr Stephen Bernard LAMBERT
Chair of the Scientific Advisory Committee for National Centre for Immunisation Research & Surveillance [133].
Has been involved in influenza and pertussis safety studies where he received grants from Sanofi and Seqirus [56].
Prof Kristine MACARTNEY
Director of National Centre for Immunisation Reseasrch & Surveillance (NCIRS) [39]. Refer to NCIRS section for detailed COI.
Presentation at "International Vaccine conference", where travel was sponsored [3].
Ex-officio Member of ATAGI [1]. Refer to ATAGI section for detailed COI.
In 2018, member of ATAGI's "Influenza Working Party" which prepares advice for ATAGI [30].
Member of ATAGI’s "Pertussis Working Party" which prepare advice on the epidemiology of pertussis and potential vaccination strategies in Australia [31].
Member of ATAGI’s "HPV working Party" which prepare advice on the epidemiology of HPV and potential vaccination strategies in Australia [40].
Member of ATAGI's COVID-19 Working Group [148] which provides advice to the Minister for Health on the immunisation program for COVID-19 vaccines as they become available in Australia.
Prof Helen MARSHALL
ATAGI member at the time, Helen Marshall was also Lead Investigator on a GlaxoSmithKline funded Bexsero meningococcal B vaccine trial in South Australia [14] & [15].
Member of the Scientific Advisory Committee for National Centre for Immunisation Research & Surveillance [133].
Researcher on clinical trials funded by vaccine manufacturer GSK, Novartis, Pfizer, Merck, Novovax, and Sanofi [4], [56].
Member of Friends of Science in Medicine [95], which supported "No Jab, No Pay" bill at the Senate Hearing in Nov 2015 [16].
Prof Peter MCINTYRE
Former Director of National Centre for Immunisation Reseasrch & Surveillance 2005 to 2017 [47]. Refer to NCIRS section for detailed COI.
Ex-officio member of ATAGI from 1999-2017 and from 2005 to 2017 National Immunisation Committee (responsible for implementation of immunisation programs) [149].
Member of ATAGI’s "Pertussis Working Party" which prepared advice on the epidemiology of pertussis and potential vaccination strategies in Australia [31].
In 2018 Member of the "Influenza Working Party" which prepared advice for ATAGI [30].
In 2003, Prof McIntyre was involved with the inception of HPV vaccination in Australia. He was instrumental in coordinating the meeting with Julia Brotherton which was funded by vaccine manufacturers GSK and CSL [48].
The ATAGI Declarations of Interest document from 2015 states that Prof McIntyre has been associated with grant funding from GSK, Pfizer, Merck, National Health & Medical Research Council and ARC [4].
Prof Jodie McVERNON
In 2018 Member of the "Influenza Working Party" which prepares advice for ATAGI [30].
Member of ATAGI’s "Pertussis Working Party" which prepare advice on the epidemiology of pertussis and potential vaccination strategies in Australia [31].
Director of Influenza Specialist Group [2], which received funding from vaccine manufacturers Abbott, GSK, Seqirus, Pfizer, Roche and Sanofi [69].
Received funding from the Australian Research Council (ARC) for vaccine clinical trial investigations [2].
Travel covered to attend a number of workshops and symposiums sponsored by vaccine companies [2].
Investigator on clinical trials funded by vaccine manufacturers GlaxoSmithKline, bioCSL, Novartis and Pfizer [2].
Member of Asia-Pacific Alliance for the Control of Influenza (APACI) [32], which has received funds from vaccine manufacturers IFPMA, Roche and Seqirus/CSL [34].
Jodie McVernon is currenty part of the Peter Doherty Institute for Infection & Immunity, COVID-19 Research team [139].
Member of ATAGI's COVID-19 Working Group [148] which provides advice to the Minister for Health on the immunisation program for COVID-19 vaccines as they become available in Australia.
Prof Terry NOLAN
Currently Head of Vaccination and Immunisation Research Group (VIRGo), which he established in the 1990’s [57] at the Murdoch Children’s Research Institute, which has received funding from vaccine manufacturers GSK, Janssen, Merck, Novavax, Sanofi and Sequiris [71].
In 2005 to 2014, Terry Nolan was appointed Chair of the Australian Technical Advisory Group on Immunisation (ATAGI)[58].
He was also appointed deputy-chair of the National Health and Medical Research Council for over 9 years[57] which decides the area of government research that should be funded [116].
In 2009 (during his employment with ATAGI [58]), Terry Nolan was the Lead Author on CSL’s funded Panvax (mono-valent H1N1) Influenza vaccine, trialled on 400 children [51]. He was also on the CSL Limited vaccine advisory board [51].
In 2010, CSL’s Fluvax (tri-valent) Influenza vaccine was suspended for its use on children 6 months to 5 years due to its increase of febrile reactions following vaccination [129]. The government inquiry was led by Terry Nolan and Peter Richmond [50] who were both on CSL’s advisory board [51]. CSL was given a $131 million federal government contract to produce the nation's entire stockpile of swine flu vaccine in 2009, half of which had to be destroyed when it passed its use-by date [7].
Terry Nolan has served on a data safety monitoring board for GSK for the HPV vaccine and GSK advisory boards (unpaid) for pertussis vaccine (2014 and 2016) [59].
Contributor of the World Health Organisation (WHO), Scientific Advisory Group of Experts (SAGE) meetings on evaluation of pandemic influenza vaccines in clinical trials each year from 2006-2011 [60].
Terry Nolan is currenty part of the Peter Doherty Institute for Infection & Immunity, COVID-19 Research team [139].
In 2010, Terry Nolan published a report “The Australian model of immunization advice and vaccine funding”, where he states that industry sponsored vaccines are generally not considered a conflict requiring exclusion [62].
Dr Rodney PEARCE
Chairman of the Immunisation Coalition [37], which is funded by vaccine manufacturers Pfizer, CSL/Seqirus, GlaxoSmithKline, Sanofi and Merck, and Google [73].
Member of the Asia-Pacific Alliance for the Control of Influenza (APACI) [32] which has received funds from vaccine manufacturers IFPMA, Roche and Seqirus/CSL [34].
Member of the Working Group on a GlaxoSmithKline funded Bexsero meningococcal B vaccine trial in South Australia [15].
Prof Peter RICHMOND
Head, Vaccine Trials Group of Telethon Kids [86], which is partnered with Johnson & Johnson, Roche, Pfizer, Novartis, GSK and Sanofi [88].
A member of the Influenza Specialist Group [49], a government body which received funding from vaccine manufacturers Abbott, GSK, Seqirus, Pfizer, Roche and Sanofi [69].
Member of the Scientific Advisory Committee for National Centre for Immunisation Research & Surveillance [133]. Refer to their section for COI.
Was Deputy Chair of ATAGI on Immunisation 2006 to 2004 [49]. Refer to ATAGI section for etailed COI.
Was Chair of ATAGI "MMR Varicella and Zoster Vaccine working Party", 2006-2014 [49].
Member of Immunisation Coalition [70], which is funded by vaccine manufacturers Pfizer, CSL/Seqirus, GlaxoSmithKline, Sanofi and Merck, and Google [73].
Has been a representative on a CSL vaccine advisory board at various times and has received nominal payments from vaccine manufacturer CSL (honoraria) [50].
Was also an investigator on the CSL funded clinical trial for Panvax vaccine in 2009 [51].
As at 2019, Chair of WA Immunisation Scentific Advisory Group [49] and Chair of WA Vaccine Safety Advisory Committee [49].
He was co-author of the Western Australian Influenza Vaccine Efficacy trial (WAIVE) for children in WA (2008 –2015) [135] which at the time was funded by vaccine manufacturers CSL, Sanofi–Pasteur and WA Health Dept in 2008 (announced by Government of Western Australia, Department of Health, Media Release 15th Feb 2008, Free vaccines to help fight child influenza - which is no longer available). However, WAVE trial is currently with Telethon Kids [136], which is partnered with Johnson & Johnson, Roche, Pfizer, Novartis, GSK and Sanofi [88].
Served on scientific advisory boards for Sanofi (influenza vaccine), GSK (maternal immunisation), AstraZeneca (influenza vaccine) [56].
Served on vaccine scentific advisory groups for GlaxsoSmith Kline (pertussis), Pfizer (pneumococcal conjugate vaccines), Baxter (mengingococcal C conjugate & ross river virus vaccine) and Murdoch Children's Research Institute (rotavirus RV3 D5MB) [49].
Principal Investigator of industry sponsored multi-centre studies for Baxter, CSL, GlaxsoSmithKline, Medimmune, Merck, Pfizer, Sanofi and Novartis [49].
Received research funding for investigator initiated studies from GlaxoSmithKline, Merck, and Novartis [49].
Prof Bruce ROBINSON
Chairperson of the Council of National Health & Medical Research Council (NHMRC) [10], who was formerly on the Advisory Board for AstraZeneca and Bayer Australia [11]. The Immunisation Handbook is approved NHMRC [12].
Kim SAMPSON
CEO of the Immunisation Coalition [38], which is funded by vaccine manufacturers Pfizer, CSL/Seqirus, GlaxoSmithKline, Sanofi and Merck, and Google [73].
Executive Director of the Asia-Pacific Alliance for the Control of Influenza (APACI) [33] which has received funds from vaccine manufacturers IFPMA, Roche and Seqirus/CSL [34].
Dr Tom SNELLING
Member of PBS/PBAC [63], which receives funds from Cost Recovery Fees & Charges from vaccine manufacturers [84].
Member of ATAGI [1]. Refer to ATAGI section for COI.
Member of the Scientific Advisory Committee for National Centre for Immunisation Research & Surveillance [133].
Head, Infectious Disease Implementation at Telethon Kids [87], which is partnered with vaccine manufacturers GSK, Johnson & Johnson, Novartis, Pfizer, Roche, and Sanofi [88].
Former investigator on pneumococcal vaccine for the National Immunisation Program [3].
Investigator on Pertussis vaccine [3].
Member of ATAGI's COVID-19 Working Group [148] which provides advice to the Minister for Health on the immunisation program for COVID-19 vaccines as they become available in Australia.
Prof Paul VAN BUYNDER
Member of the Immunisation Coalition [70], which is funded by vaccine manufacturers Pfizer, CSL/Seqirus, GlaxoSmithKline, Sanofi and Merck, and Google [73].
In January 2020 he expressed "pushing anti-vax views should be criminal offence" [150].
AUSTRALIAN INFLUENCE
NEWS CORP AUSTRALIA
News Corp Aust tabloids were behind the "No Jab, No Play" media campaign [79], which was obligingly adopted as policy by politicians across the political spectrum in Australia, and enacted as the coercive "No Jab, No Pay" law under Malcolm Turnbull as Prime Minister in January 2016.
In 1954, Rupert Murdoch took control of News Limited in Australia [98].
News Corp Aust is a corporate partner of the Murdoch Children's Research Institute [78], an organisation which is involved in vaccine product research and development, which has received funds from Vaccination and Immunisation Research Group which has received funds from vaccine manufacturers GSK, Janssen, Merck, Novavax, Sanofi and Sequiris [71].
FRIENDS OF SCIENCE IN MEDICINE (FSM)
Is affiliated with Australian Skeptics [96].
President of FSM is Ken Harvey [132].
Members include Helen Marshall, Ian Frazer, Peter Doherty, Robert Booy and Stephen Lambert [95] who have conflicts of interest with vaccine manufacturers. Refer to their sections for COI.
Member David Hawkes [95] is also a member of SAVN [92]. Refer to his section for COI.
Member John Cunningham [95] and Executive Member Sue Ieraci [128], were influential at the "No Jab, No Pay" Senate Inquiry Hearing in 2015 [16].
STOP THE AUSTRALIAN VACCINATION NETWORK (SAVN)
A coercive vaccination lobby group [99].
People associated with SAVN are Administrators David Hawkes, John Cunningham and Patrick Stokes [128] and Rachael Dunlop [92], who were main participants at the "No Jab, No Pay" Senate Inquiry Hearing in 2015 [97]. Refer to David Hawkes section for COI.
THE FUTURE FUND
CSL shares valued at $674 million, plus Pfizer shares worth more than $265 million, $251 million of Johnson and Johnson shares, and over $134 million of shares in Merck were equity holdings of the Future Fund Board of Guardians as at 30 June 2019 [100].
$310 million worth of Facebook shares are also listed in the Future Fund portfolio (as at 30 June 2019) [100], and it is notable that Facebook is now hindering Australians free discussion of matters relevant to vaccine products and policy with its self-appointed 'fact-checkers'.
INTERNATIONAL INFLUENCE
BILL & MELINDA GATES FOUNDATION (B&MGF)
Bill and Melinda Gates have been involved in vaccine promotion since 1998, with the creation of their Bill & Melinda Gates Children’s Vaccine Programme [101].
The Gates Foundation is a founding partner of Global Alliance for Vaccines and Immunization (GAVI) [102]. GAVI is partnered with vaccine manufacturers [103].
In 2002, the Wall Street Journal reported that the B&MGF purchased shares of nine Big Pharma companies, valued at nearly$205 million [104].
A publication in The Nation found "close to $250 million in charitable grants from the Gates Foundation to companies in which the foundation holds corporate stocks and bonds: Merck, Novartis, GlaxoSmithKline, Vodafone, Sanofi, Ericsson, LG, Medtronic, Teva" [105].
In 2013, Dr Penny Heaton was appointed Director of Vaccine Development within B&MGF's Global Health Program, and previously worked on vaccine development at Merck and Novartis [106].
In 2011, Trevor Mundel was appointed President of B&MGF, and previously served in leadership positions working on vaccine development at Novartis, Pfizer and Parke-Davis [107].
In 2006, Dr Tachi Yamada was appointed Executive Director of B&MGF's Global Health Program, who simultaneously was employed as chairman of Research and Development at GSK [108].
In 2009, Kate James was appointed Chief Communications Officer at B&MGF, who previously worked at GSK for almost 10 years [109].
GLOBAL ALLIANCE FOR VACCINES & IMMUNIZATIONS (GAVI)
Gates Foundation pledged US$ 750 million to set up GAVI in 1999. The Foundation is a key GAVI partner in vaccine market shaping [110].
GAVI helps vaccinate almost half the world’s children. GAVI is partnered with The World Health Organisation (WHO), Bill & Melinda Gates Foundation, UNICEF, The World Bank and Vaccine Manufacturers [103].
Representatives of the International Federation of Pharmaceutical Manufacturers & Associations (IFPMA) have one seat on the GAVI Board. The IFPMA represents more than 55 members of national industry associations, including Johnson & Johnson, GlaxoSmithKline, Merck & Co., Novartis, Sanofi Pasteur, the vaccines division of Sanofi-Aventis and Pfizer [111].
GLOBAL VACCINE ACTION PLAN (GVAP)
The GVAP was endorsed by the WHO in 2012 to prevent millions of deaths by 2020 through more equitable access to existing vaccines for people in all communities [112].
Their leadership is of the Bill & Melinda Gates Foundation, GAVI Alliance, UNICEF, United States National Institute of Allergies and Infectious Diseases and WHO, along with all partners – governments and elected officials, health professionals, academia, manufacturers, global agencies, development partners, civil society, media and the private sector – are committed to achieving the ambitious goals of the GVAP [112]. Note: “Pfizer has been a longstanding supporter of UNICEF (since 1998) through in-kind donations, corporate contributions, workplace-giving programs, and special events.” [146].
WORLD HEALTH ORGANISATION (WHO)
The WHO’s Strategic Advisory Group of Experts on Immunization, (SAGE), is the principal advisory group to the WHO for vaccines and immunization, advising the WHO on overall global policies and strategies [113].
In 2016, members of SAGE include Terry Nolan [60] and Andrew Pollard [61]. Refer to their names for COI.
The WHO has received funding in 2020 USD$832M from Bill & Melinda Gates Foundation and USD$372M from GAVI [114], their COI as outlined.
Juhani Eskola, a Finnish vaccines adviser on the WHO board, has received £5.6m (€6.2m; $9m) for his research centre, the Finnish National Institute for Health and Welfare from vaccine manufacturer GSK [115].
ANDREW POLLARD Prof
Chief Investigator of the Bexsero meningococcal B vaccine trials [117], previously owned by Novartis and now owned by GSK.
Chair of The Joint Committee on Vaccination & Immunisation (JCVI) [118], who advises UK health departments on immunisation.
Director of the Oxford Vaccine Group (OVG) [119], which received funding from vaccine manufacturers for their trials [120].
Professor of Paediatric Infection and Immunity at the University of Oxford, which has significant financial influence from Vaccine Manufacturing and Innovation Centre UK [121].
Was chair of the European Medicines Agency, Scientific Advisory Group of Vaccines (ASAGV) [122], whose main stakeholders are pharmaceutical industries [123].
In 2016, became a member of the World Health Organisation (WHO) Scientific Advisory Group of Experts (SAGE) [61].
Chaired the UK’s National Institute for Health and care Excellence (NICE) meningitis guidelines development group [61].
Developer in AstraZeneca Oxford COVID-19 Vaccine [124].
This document is sourced from the website of the Informed Medical Options Party.
You can see the original document, with all the footnotes live and an option to contribute further documentation here. Footnote numbers have been retained above for ease of reference.
The group calls for accountability from the Australian government as follows:
Australians deserve responsible Government. We will shine a spotlight in places where accountability is lacking by:
Supporting the call for a Royal Commission into the Government’s COVID response, including a review of the roles played by the Therapeutic Goods Administration (TGA), the Gene Technology Regulator, the Australian Health Practitioners Regulation Authority (AHPRA), Health Care Complaints Commission (HCCC) and any other organisation whose decisions impacted the health and freedoms of our citizens.
Challenging Australia’s inclusion in the World Health Organisation (WHO) and the United Nations (UN). While the WHO and the UN may provide consultative advice, ultimately, the responsibility to independently assess and make decisions lies with our elected representatives. All current foreign trade agreements should be reassessed.
Empowering health professionals to gain true informed consent (through advising their patients of all the side effects, risks and benefits) without fear of repercussion or reprisal, prior to administering any medical product or procedure. Directives from any governing body that contradicts or interferes with this process, or censors health professionals, should be void and subject to penalties. Any health professional who fails to gain informed consent should be penalised.
Challenging the immunity from liability granted to pharmaceutical companies against prosecution for vaccine harm.
Calling for a Royal Commission into claims that Australians have died or been harmed by vaccinations.
Calling for any person or organisation that is found to have purchased a patent for the purpose of preventing a science or technology from being operational to be penalised. The purpose of this policy is to make it an offence to purchase a patent (for example, energy, health and life saving technologies) only for the purpose of shelving the invention and preventing others from ever using it.